Systematic Reviews Explained Amstarã¢â‚¬â€how to Tell the Good From the Bad and the Ugly
- Research article
- Open up Admission
- Published:
A methodological quality assessment of systematic reviews and meta-analyses of antidepressants effect on depression dorsum pain using updated AMSTAR
BMC Medical Research Methodology volume 20, Article number:14 (2020) Cite this article
Abstract
Background
Antidepressants are prescribed widely to manage low back pain. There are a number of systematic reviews and meta-analyses which have investigated the efficacy of the treatments, while the methodological quality of them has not been assessed yet. This study aims to evaluate the methodological quality of the systematic reviews and meta-analyses investigating the effect of antidepressants on depression back pain.
Methods
A systematic search was conducted in PubMed, EMBASE, Medline, and Cochrane Library databases upward to November 2018. The 16-item Assessment of Multiple Systematic Reviews (AMSTAR2) scale was used to assess the methodological quality of the studies. Systematic reviews and meta-analyses of the Antidepressants handling effects on low back pain published in English were included. There was no limitation on the type of Antidepressants drugs, clinical setting, and study population, while non-systematical reviews and qualitative and narrative reviews were excluded.
Results
A total of 25 systematic reviews and meta-analyses were evaluated; the studies were reported between 1992 and 2017. Obtained results from AMSTAR2 showed that 11 (44%), 9 (36%) and 5 (20%) of the included studies had high, moderate and depression qualities, respectively. 13(52%) of studies assessed risk of bias and 2(xx%) of meta analyses considered publication bias. Also, 16 (64%) of the included reviews provided a satisfactory caption for any heterogeneity observed in the results.
Conclusions
Although the trend of publishing high quality papers in ADs effect on LBP increased recently, performing more high-quality SRs and MAs in this field with precise subgroups of the type of pains, the grade of drugs and their dosages may give clear and more reliable prove to help clinicians and policymakers.
Introduction
Depression dorsum pain (LBP) is a major cause of inability. It was ranked first and sixth in terms of disability (YLDs) and overall brunt (DALYs), respectively [1]. Pharmaceutical and non-pharmaceutical therapies are taken extensively to tackle this issue; in this fashion, guidelines provide a variety of suggested medicines and practices such as the utilise of nonsteroidal anti-inflammatory drugs (NSAIDs) and weak opioids in patients with non-specific/acute LBP for short periods [2,3,iv,v]. Although antidepressants (ADs) are not recommended as the first-line prescribed medicine to manage LBP, they are taken widely [2, half-dozen,7,8,9]. There is conflicting evidence about the issue of antidepressant, unlike studies showed their beneficial role in pain reduction while others have opposed them due to the high risk of adverse furnishings such every bit dry oral cavity, dizziness, nausea, headache, and constipation and no clear evidence of efficacy [ten,11,12,13]. In addition, some systematic reviews (SRs) and meta-analyses (MAs) which summarized the results of the available evidence, provided heterogeneous results which make information technology hard to decision regarding the efficacy of ADs [14,xv,xvi,17,18].
An SR is a type of literature review which critically evaluates research studies. Information technology can summarize results obtained from a plethora of studies helping researchers and clinicians to keep upwards with the new findings. MA is also a statistical arroyo to summarize the evidence extracted from secondary information obtained from the SR of studies in a specific field of study. SRs and MAs provide a reference source for aiding experts in conclusion making. Despite their rapid growth and profound influence in health science, discrepancies of the results in studies on the same subject has made them unreliable in decision making. One reason is the affair of methodological quality of the reviews [xix,twenty,21]. In this respect, evaluating the reliability and methodological quality of the studies is of great importance. In that location are some technical and methodological approaches to enrich SRs and MAs in order to achieve valid results [22,23,24,25,26]. For this purpose, the Assessment of Multiple Systematic Reviews (AMSTAR) scale provides an appraisal tool for measuring the methodological quality of SRs [27, 28]. The purpose of this study was to assess the methodological quality of SRs and MAs of the office of ADs in treating LBP using the updated version of AMSTAR.
Materials and methods
Data sources and study selection
We searched for all SRs and MAs up to Nov 2018 using the PubMed, EMBASE, Medline, and Cochrane Library databases. Our search strategy followed the recommendations of the Cochrane Back Review Group [22,23,24]. Combinations of the following keywords were used in the search: "low back pain" AND "chronic low back pain" AND "not-specific low dorsum pain" AND "sciatica" AND "leg pain" AND "antidepressant" AND ("TCA" OR "tricyclic antidepressants") AND ("SSRI" OR "selective serotonin reuptake inhibitors") AND ("SNRI" OR "serotonin and norepinephrine reuptake inhibitors") AND ("TeCA" OR "tetracyclic antidepressants") AND "meta-assay" AND "systematic review". The text words and MeSH terms were entered depending on the databases characteristics. The reference lists from retrieved articles were also screened for additional applicable studies.
Inclusion and exclusion criteria
Nosotros included SRs and MAs of the ADs treatment effects on LBP published in English language language. We also included all types of low back pain such as Chronic Low Back Pain (CLBP), Non-specific Low Back Pain (NLBP), Chronic Not-specific Low Dorsum Pain (CNLBP) and sciatica, regardless of the crusade of pain such every bit cancer, fracture, inflammatory disease, etc. At that place was no limitation on the type of ADs drugs, clinical setting, and study population, while non-systematical reviews and qualitative and narrative reviews were excluded.
Study selection and data extraction
Screening of titles and abstracts of the retrieved studies for inclusion was conducted past two independent reviewers (RBY and MHP). The total texts of the eligible reviews were extracted and evaluated to decide whether they met the inclusion criteria by RBY and MHP. Any disagreements were resolved by consensus through give-and-take and the third person (FRT). For each study, the following information was extracted: authors, year of publication, study design, blazon of study and intervention, characteristics of study population, outcome measurement and summary of obtained 50 results. PRISMA flow diagram [29] was used to guide the process of inclusion and exclusion of studies.
Assessment of methodological quality of included studies
Quality assessment was performed independently by two authors (RBY and MHP). Any discrepancies were resolved past discussion, and a blinded third reviewer was consulted if necessary. We used the updated Assessment of Multiple Systematic Reviews (AMSTAR2) appraisement tool to evaluate the methodological quality of eligible SRs and MAs [28]. It has some advantages compared to its previous version, such as the inclusion of not-randomized studies in SRs, and a different scoring arrangement which helps reduce bias produced by quality scores obtained traditionally by summing up scores and getting an overall score [30]. AMSTAR2 contains 16 items; i.e., four domains have been added to this new version of AMSATR. Ii of these were adopted directly from the ROBINS-I tool, namely, elaboration of the PICO and the way in which hazard of bias was handled during testify synthesis. Some other one was the discussion of possible causes and significance of heterogeneity. The last new domain was the justification of selection of study designs to deal with non-randomized designs. The domain-specific questions in AMSTAR 2 are framed and then that a "Aye" reply denotes a positive result. "Not Applicative" and "Cannot Answer" options in the original AMSTAR instrument were removed and "Partial Aye" responses accept been provided where it is worthwhile to place fractional adherence to the standard. Moreover, the AMSTAR tool has a good agreement, reliability, construct validity, and feasibility to assess the quality of systematic reviews [31].
Data analysis
Characteristics of the studies are reported in Table 1. In addition, Tables 2 and 3 bear witness the results of AMSTAR2 domain ("Aye", "Partial Yes", "No") of each included written report. Moreover, the secular trend of the number and quality of included reviews was illustrated as well.
Results
Study identification
Through the initial search, we extracted 3700 potentially relevant articles past searching electronic databases and other resources. Afterward skimming the titles and abstracts and identifying duplications, 3646 articles were excluded. The total texts of the remaining 54 articles were read carefully in their entirety. Xx-five articles were eligible for the inclusion; 29 Narrative/reviews were excluded from the assessment. All included studies were SRs and MAs on the role of ADs in LBP. The PRISMA flowchart guided the pick process of extracted literature (Fig. 1).
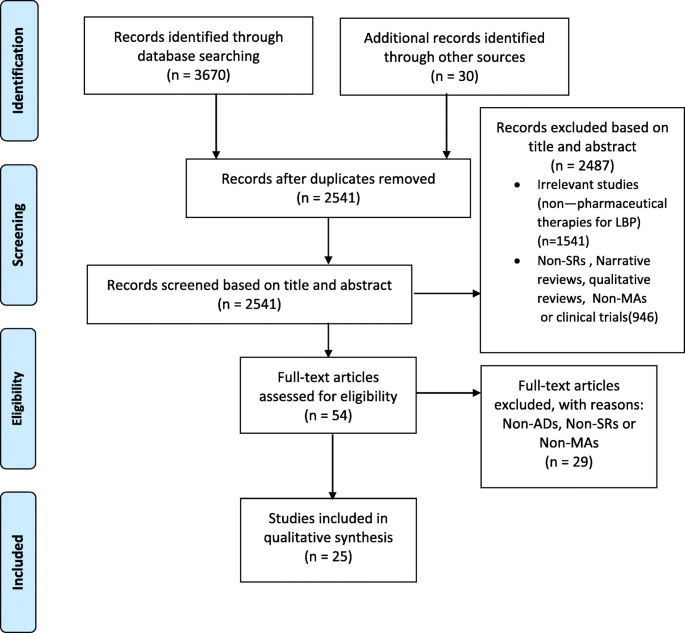
PRISMA Menses Diagram of the Review Search and Identification
Characteristics of included SRs
Characteristics of the 25 SRs and MAs are presented in Tabular array 1. Studies were reported between 1992 and 2017. The number of studies included in MAs ranged from 4 to 10 intervention studies on ADs. Studies included were performed on relatively homogeneous patients or populations which endure from chronic depression dorsum pain (CLBP), non-specific low back pain (NLBP), chronic non-specific low back pain (CNLBP) and sciatica. Moreover, multiple Advertizement drug categories with different dosages were considered as intervention. Six out of 25 included studies had no specific subgroups of drug intervention; others consisted of selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), tetracyclic antidepressant (TeCA), selective serotonin reuptake inhibitors (SSRIs), and serotonin-norepinephrine reuptake inhibitors (SNRIs). Regarding study design, most studies included in MAs or SRs were randomized controlled trials. In add-on, nosotros reported the results of the AMSTAR quality cess of each written report.
Assessment of methodological quality of included SRs
The assessments of the methodological quality are given in Tables 2 and three. Out of 25 included studies, 11, nine and 5 studies were classified equally loftier [2, 14, 16, 17, 33, 34, 37, 41, 44, 45, 48] moderate [12, 35, 36, 42, 43, 47, 49] and low [32, 38,39,40, 46] quality, respectively.
Tabular array iii shows the results of the methodological quality cess according to each item. Items i: "Did the inquiry questions and inclusion criteria for the review include the components of PICO (population, intervention, control grouping and outcome)?", iii: "Did the review authors explicate their option of the written report designs for inclusion in the review?", 8: "Did the review authors describe the included studies in adequate particular?", 10: "If meta-assay (MA) was justified did the review authors utilize advisable methods for statistical combination of results?", 11: "If meta-assay (MA) was justified did the review authors use appropriate methods for statistical combination of results?" and 16: "Did the review authors written report any potential sources of conflict of interest, including any funding they received for conducting the review?" were the most common AMSTAR items in which the studies scored highest, while they lost points in ii: "Did the report of the review contain an explicit argument that the review methods were established prior to conduct of the review and did the report justify whatever meaning deviations from the protocol?" and 15: "If they performed quantitative synthesis did the review authors conduct out an adequate investigation of publication bias (small written report bias) and discuss its likely impact on the results of the review?". For items ix, 12, 13 and fourteen which were related to the event of Chance of Bias (RoB) and heterogeneity, they got an boilerplate score. 13 (52%) of the studies used a satisfactory technique for assessing the RoB; the Cochrane Collaboration's tool was the about common tool applied. v (fifty%) of MAs assessed the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis. 14 (56%) of the review studies accounted for RoB in private studies when interpreting the results of the review and 16 (64%) of them provided a satisfactory caption for, and discussion of, any heterogeneity observed in the results of the review. Only two (xx%) of the meta-analyses out of ten carried out an adequate investigation of publication bias (small study bias) and discussed its likely bear upon on the results of the review. Tendency analysis showed that since 2016 an increasing trend was observed with regard to the number of publications in this topic with high quality (Fig. 2).
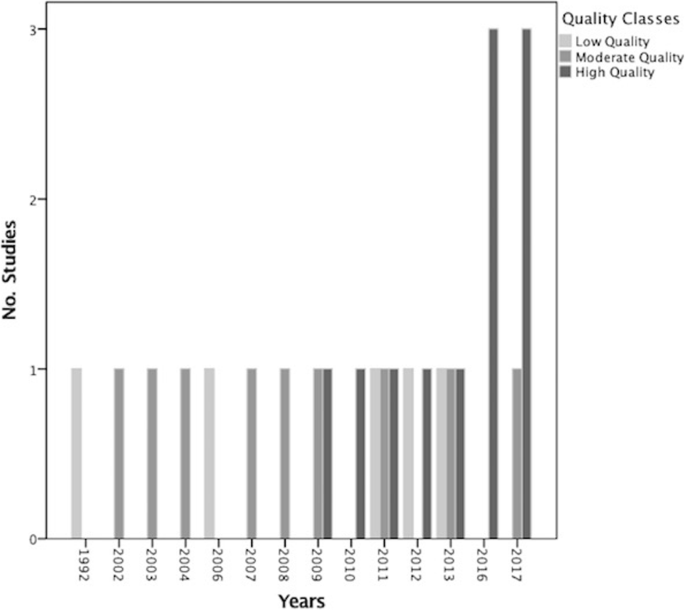
The secular trend of the number and quality of included reviews
Discussion
Methodological quality assessment
To the best of our knowledge, this is the offset report to examine specifically the quality of SRs and MAs on the effectiveness of ADs on LBP using AMSTAR 2. In our study, xi (44%), 9 (36%) and v (20%) studies were classified every bit high, moderate, and low quality, respectively. The former version of AMSTAR assigns fifty-fifty weights to each detail and produces an overall score while it is subjected to bias estimation. To overcome this issue, AMSTAR 2 has been designed in a way that it does non estimate an overall score. A loftier score may disguise disquisitional weaknesses in specific domains, such as an inadequate literature search or a failure to assess the take a chance of bias (RoB). RoB is a disquisitional section of the appraisal of any systematic reviews. It was conducted by 13 (52%) of the studies which mostly applied the Cochrane Collaboration'south tool. 8 (32%) of the studies assessed quality instead of RoB; nosotros mention them also to distinguish studies which did none. Contrary to the AMSTAR which focused on the quality assessment of included studies (Particular 7), AMSTAR 2 considered RoB in three items [9, 12, 13]. A study may have the highest possible quality and even so have an important risk of bias. For instance, in many situations, it is impractical or incommunicable to blind participants or study personnel to the intervention group. The Newcastle Ottawa Scale, SIGN, and the Mixed Methods Appraisal Tool as well every bit Cochrane instrument and ROBINS-I are the nearly comprehensive instruments for assessing RoB. It is important that the impact of RoB exist considered in the results of the MAs, so they should assess the touch of this by meta-regression analysis, or by estimating pooled outcome sizes by excluding studies at loftier RoB through sensitivity assay. 16 (64%) of the included reviews provided a satisfactory explanation for whatsoever heterogeneity observed in the results. Equally a affair of fact, heterogeneity in the SRs and MAs points to the variation in study outcomes betwixt studies. Considering potential sources of heterogeneity which can be related to the domains of bias or PICO description (population, intervention, control group and effect) is essential. Assessing heterogeneity through Chi-squared test or I-squared index and conducting appropriate methods of analysis similar Stock-still/Random-result models and other methods such equally meta-regression and sensitivity analysis help find sources of heterogeneity and strengthen the results. In addition, 2 (20%) of the included MAs carried out an adequate investigation of publication bias and discussed its likely touch on the results. Methods of exploring publication bias in MAs such equally funnel plot, Egger and Begg's test, etc., were presented [50,51,52,53]. In addition, the secular trend of studies showed that since 2007 which was the initiation of AMSTAR more than publications at moderate to high quality were published and since 2016 nigh of them were high-quality. It showed that authors were more enlightened of items which can improve the quality of their research and consequently provide more precise and reliable results.
Summary of ADs effect on LBP
Near SRs and MAs in this area, illustrated that there was no articulate testify of ADs effectiveness on LPB [2, 16, 34, 41, 54,55,56] while others achieved contradictory results [eighteen, 35, 36, 57, 58]. Some of them showed that TCAs had pregnant analgesic event more than other types of Ads [15, 17, 32, 40, 42, 59,60,61,62], while there exists contradiction every bit well [63]. In add-on, some reviews reported a lack of sufficient data for the decision [33, 55, 64]. Significant side effects were observed in ADs as well [ii, 12, 15, eighteen].
Strengths and limitations
The present study is the first to comprehensively assess the methodological quality of SRs on the effect of ADs on LBP. We used the updated version of AMSTAR appraisal tools (AMSTAR 2) which has some merits over the older version. This evaluation tin can help experts to rely on high-quality studies when getting stuck in the dilemma of alien literature. A limitation of our written report was that it only included reviews published in English, so publication bias could be introduced.
Determination
Although the tendency of publishing loftier quality papers in ADs effect on LBP increased recently, performing more than high-quality SRs and MAs in this field with precise subgroups of the blazon of pains, the grade of drugs and their dosages may give articulate and more than reliable testify to help clinicians and policymakers.
Availability of data and materials
All data generated or analyzed during the current study are included in this published article.
Abbreviations
- ADs:
-
Antidepressants
- AMSTAR:
-
Assessment of Multiple Systematic Reviews
- CLBP:
-
Chronic Depression Back Hurting
- CNLBP:
-
Chronic Non-specific Low Back Hurting
- MAs:
-
Meta-Analyses
- NLBP:
-
Not-specific Depression Back Pain
- PICO:
-
Population, Intervention, Command group and Outcome
- SNRIs:
-
Serotonin and Norepinephrine Reuptake Inhibitors
- SRs:
-
Systematic Reviews
- SSRIs:
-
Selective Serotonin Reuptake Inhibitors
- TCAs:
-
Tricyclic Antidepressants
- TeCA:
-
Tetracyclic Antidepressant
References
-
Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, et al. The global burden of low back pain: estimates from the global brunt of disease 2010 study. Ann Rheum Dis. 2014;73(6):968.
-
National GCU. Low Back pain and sciatica in over 16s: assessment and management: National Institute for health and care excellence (UK); 2016.
-
Wong J, Cote P, Sutton D, Randhawa M, Yu H, Varatharajan S, et al. Clinical practise guidelines for the noninvasive management of low back pain: a systematic review past the Ontario protocol for traffic injury management (OPTIMa) collaboration. Eur J Pain. 2017;21(2):201–16.
-
Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer Thousand, et al. Nonpharmacologic therapies for depression back hurting: a systematic review for an American College of Physicians Clinical Do Guideline. Ann Intern Med. 2017;166(seven):493–505.
-
Oliveira CB, Maher CG, Pinto RZ, Traeger Air conditioning, Lin C-WC, Chenot J-F, et al. Clinical practice guidelines for the direction of non-specific low dorsum hurting in primary care: an updated overview. Eur Spine J. 2018;27(11):2791–803.
-
Di Iorio D, Henley East, Doughty A. A survey of primary care medico practice patterns and adherence to astute depression back problem guidelines. Arch Fam Med. 2000;ix(10):1015.
-
Fishbain D. Evidence-based data on pain relief with antidepressants. Ann Med. 2000;32(5):305–sixteen.
-
Koes BW, van Tulder MW, Ostelo R, Kim Burton A, Waddell Thousand. Clinical guidelines for the Management of low Back Pain in chief care: an international comparison. Spine. 2001;26(22):2504–13.
-
Orsulak PJ, Waller D. Antidepressant drugs: additional clinical uses. J Fam Pract. 1989;28:209–sixteen.
-
Dickens C, Jayson M, Sutton C, Creed F. The relationship between hurting and depression in a trial using paroxetine in sufferers of chronic low dorsum pain. Psychosomatics. 2000;41(6):490–nine.
-
Katz J, Pennella-Vaughan J, Hetzel RD, Kanazi GE, Dworkin RH. A randomized, placebo-controlled trial of bupropion sustained release in chronic low dorsum pain. J Pain. 2005;6(ten):656–61.
-
Riediger C, Schuster T, Barlinn Chiliad, Maier S, Weitz J, Siepmann T. Adverse effects of antidepressants for chronic Hurting: a systematic review and Meta-analysis. Front Neurol. 2017;viii:307.
-
Atkinson JH, Slater MA, Capparelli EV, Wallace MS, Zisook S, Abramson I, et al. Efficacy of noradrenergic and serotonergic antidepressants in chronic dorsum pain: a preliminary concentration-controlled trial. J Clin Psychopharmacol. 2007;27(2):135–42.
-
Chou R, Deyo R, Friedly J, Skelly A, Weimer M, Fu R, et al. Systemic pharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166(7):480–92.
-
Chou R, Huffman LH. Medications for astute and chronic low back pain: a review of the evidence for an American hurting society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):505–14.
-
Kuijpers T, van Middelkoop M, Rubinstein S, Ostelo R, Verhagen A, Koes B, et al. A systematic review on the effectiveness of pharmacological interventions for chronic not-specific low-back hurting. Eur Spine J. 2011;20(i):twoscore–50.
-
van den Driest JJ, Bierma-Zeinstra SM, Bindels PJ, Schiphof D. Amitriptyline for musculoskeletal complaints: a systematic review. Fam Pract. 2017;34(two):138–46.
-
Chung J, Zeng Y, Wong T. Drug therapy for the handling of chronic nonspecific depression back pain: systematic review and meta-analysis. Pain Physician. 2013;16(6):E685–704.
-
Moher D, Soeken Thou, Sampson M, Ben-Porat L, Berman B. Assessing the quality of reports of systematic reviews in pediatric complementary and alternative medicine. BMC Pediatr. 2002;ii(1):3.
-
Sharif MO, Janjua-Sharif F, Ali H, Ahmed F. Systematic reviews explained: AMSTAR-how to tell the good from the bad and the ugly. Oral Health Dent Manag. 2013;12(i):9–16.
-
Cornell JE, Laine C. The scientific discipline and art of deduction: complex systematic overviews. Ann Intern Med. 2008;148(ten):786–eight.
-
van Tulder 1000, Furlan A, Bombardier C, Bouter L, Group tEBotCCBR. Updated method guidelines for systematic reviews in the Cochrane collaboration Back review group. Spine. 2003;28(12):1290–9.
-
Furlan Advert, Pennick V, Bombardier C, van Tulder Thousand. 2009 updated method guidelines for systematic reviews in the Cochrane Back review group. Spine. 2009;34(eighteen):1929–41.
-
Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, et al. 2015 updated method guideline for systematic reviews in the Cochrane Back and neck group. Spine. 2015;40(21):1660–73.
-
Higgins JPTTJ, Chandler J, Cumpston 1000, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 Cochrane. 2d ed; 2019. [updated July 2019: [Available from: www.handbook.cochrane.org. Accessed 10 Sept 2018]
-
Furlan AD, Pennick V, Bombardier C, van Tulder Thou, Group ftEBotCBR. 2009 updated method guidelines for systematic reviews in the Cochrane Back review group. Spine. 2009;34(18):1929–41.
-
Shea BJ, Grimshaw JM, Wells GA, Boers Grand, Andersson Northward, Hamel C, et al. Development of AMSTAR: a measurement tool to appraise the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7(ane):x.
-
Shea BJ, Reeves BC, Wells Grand, Thuku Chiliad, Hamel C, Moran J, et al. AMSTAR 2: a disquisitional appraisal tool for systematic reviews that include randomised or not-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
-
Moher D, Shamseer L, Clarke Grand, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;iv(1):1.
-
Greenland Due south, O'Rourke K. On the bias produced past quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics. 2001;2(iv):463–71.
-
Shea BJ, Bouter LM, Peterson J, Boers M, Andersson N, Ortiz Z, et al. External validation of a measurement tool to assess systematic reviews (AMSTAR). PloS one. 2007;2(12):e1350–e.
-
Onghena P, Van Houdenhove B. Antidepressant-induced analgesia in chronic non-malignant pain: a meta-analysis of 39 placebo-controlled studies. Pain. 1992;49(2):205–19.
-
Pinto RZ, Maher CG, Ferreira ML, Ferreira PH, Hancock Yard, Oliveira VC, et al. Drugs for relief of pain in patients with sciatica: systematic review and meta-analysis. BMJ. 2012;344:e497.
-
Urquhart DM, Hoving JL, Assendelft WJ, Roland Grand, van Tulder MW. Antidepressants for non-specific low back pain. Cochrane Database Syst Rev. 2008;(1):CD001703. https://doi.org/10.1002/14651858.CD001703.pub3.
-
Machado Fifty, Kamper S, Herbert R, Maher C, McAuley J. Analgesic furnishings of treatments for non-specific low back hurting: a meta-assay of placebo-controlled randomized trials. Rheumatology. 2008;48(5):520–seven.
-
Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back hurting: a meta-analysis. Curvation Intern Med. 2002;162(i):19–24.
-
Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, et al. Noninvasive treatments for low back pain. 2016.
-
Mercier A, Auger-Aubin I, Lebeau J-P, Schuers M, Boulet P, Hermil J-Fifty, et al. Testify of prescription of antidepressants for not-psychiatric conditions in primary care: an assay of guidelines and systematic reviews. BMC Fam Pract. 2013;14(1):55.
-
Romano CL, Romano D, Lacerenza G. Antineuropathic and antinociceptive drugs combination in patients with chronic low back pain: a systematic review. Pain Res Treat. 2012;2012:154781.
-
Morlion B. Pharmacotherapy of low dorsum pain: targeting nociceptive and neuropathic pain components. Curr Med Res Opin. 2011;27(i):eleven–33.
-
Savigny P, Kuntze Southward, Watson P, Underwood Chiliad, Ritchie Grand, Cotterell M, et al. Depression back pain: early management of persistent not-specific low back pain, vol. fourteen. London: National Collaborating Centre for Primary Care and Royal Higher of Full general Practitioners; 2009.
-
Staiger TO, Gaster B, Sullivan Doc, Deyo RA. Systematic review of antidepressants in the handling of chronic low back pain. Spine. 2003;28(22):2540–5.
-
White AP, Arnold PM, Norvell DC, Ecker E, Fehlings MG. Pharmacologic Direction of Chronic low Back Pain: synthesis of the prove. Spine. 2011;36:S131–S43.
-
Cawston H, Davie A, Paget M-A, Skljarevski 5, Happich M. Efficacy of duloxetine versus alternative oral therapies: an indirect comparison of randomised clinical trials in chronic low back pain. Eur Spine J. 2013;22(nine):1996–2009.
-
Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive treatments for acute, subacute, and chronic depression back pain: a clinical do guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514–30.
-
Perrot S, Maheu E, Javier RM, Eschalier A, Coutaux A, LeBars M, et al. Guidelines for the apply of antidepressants in painful rheumatic conditions. Eur J Hurting. 2006;10(3):185.
-
Perrot Due south, Javier R-M, Marty Thousand, Le Jeunne C, Laroche F, CEDR FRS. Pain study section. Is there any prove to support the use of anti-depressants in painful rheumatological conditions? Systematic review of pharmacological and clinical studies. Rheumatology. 2008;47(8):1117–23.
-
Patetsos E, Horjales-Araujo Due east. Treating chronic pain with SSRIs: what practise nosotros know? Pain Inquiry and Management. 2016;2016:2020915.
-
Schnitzer TJ, Ferraro A, Hunsche E, Kong SX. A comprehensive review of clinical trials on the efficacy and safety of drugs for the treatment of low back pain. J Pain Symptom Manag. 2004;28(1):72–95.
-
Calorie-free RJ, Pillemer DB. Summing up: the science of reviewing research Harvard University printing: Cambridge, MA, 1984, thirteen+191 pp. Educ Res. 1986;15(8):sixteen–7.
-
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication Bias. Biometrics. 1994;fifty(4):1088–101.
-
Egger M, Smith GD, Schneider Yard, Minder C. Bias in meta-analysis detected past a simple, graphical exam. BMJ. 1997;315(7109):629–34.
-
Sterne JAC, Egger M, Smith GD. Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101.
-
Maher C, Underwood Yard, Buchbinder R. Non-specific low back pain. Lancet. 2017;389(10070):736–47.
-
Businesswoman R, Folder A, Attal N, Casale R, Dickenson A, Treede RD. Neuropathic depression back pain in clinical practice. Eur J Pain. 2016;20(half dozen):861–73.
-
Williamson OD, Sagman D, Bruins RH, Boulay LJ, Schacht A. Antidepressants in the treatment for chronic low Dorsum pain: questioning the validity of Meta-analyses. Pain Pract. 2014;14(ii):E33–41.
-
Tandon VR, Mahajan A, Singh One thousand, Sharma A, Rai H. Antidepressants/Antiepileptic drugs-Chronic Depression Dorsum hurting. Pain. 2007;2:5.
-
Maizels M, McCarberg B. Antidepressants and antiepileptic drugs for chronic not-cancer pain. Am Fam Physician. 2005;71(3):483–90.
-
Dharmshaktu P, Tayal V, Kalra BS. Efficacy of antidepressants as analgesics: a review. J Clin Pharmacol. 2012;52(ane):vi–17.
-
Sardar Yard, Rashid Chiliad, Khandoker M, Khan A. Anticonvulsants and antidepressants in chronic pain management. J Recent Adv Pain. 2016;2(3):90–3.
-
Pinto RZ, Verwoerd AJ, Koes BW. Which hurting medications are effective for sciatica (radicular leg pain)? BMJ. 2017;359:j4248.
-
Chou R, Côté P, Randhawa 1000, Torres P, Yu H, Nordin M, et al. The global spine intendance initiative: applying bear witness-based guidelines on the non-invasive management of back and neck pain to low-and eye-income communities. Eur Spine J. 2018;27:i–10.
-
Koes BW, Backes D, Bindels PJ. Pharmacotherapy for chronic non-specific depression back pain: current and future options. Adept Opin Pharmacother. 2018;19(6):537–45.
-
Mika J, Zychowska K, Makuch Westward, Rojewska E, Przewlocka B. Neuronal and immunological ground of activity of antidepressants in chronic pain–clinical and experimental studies. Pharmacol Rep. 2013;65(6):1611–21.
Acknowledgements
The authors would similar to acknowledge Dr. Amir Ghorbanpour for critical editing of English grammar and syntax of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Affiliations
Contributions
MHP (Epidemiologist), MM (Neurosurgeon), FRT (Physician) and RBY (Biostatistician) had significant contribution to the formulation, design, conquering, analysis and interpretation of the information. Methodological concepts were considered by RBY, MHP and prof. FRT, and medical concepts were critically interpreted by MM, FRT and MHP. All authors worked on the drafting and agreed on concluding approval of the version to be published. Also, agreement to be answerable for all aspects of the piece of work in ensuring that questions related to the accuracy or integrity of any role of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ideals blessing and consent to participate
Not applicative.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Boosted information
Publisher'southward Annotation
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution four.0 International License (http://creativecommons.org/licenses/past/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(southward) and the source, provide a link to the Artistic Commons license, and betoken if changes were made. The Artistic Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/cypher/i.0/) applies to the data made available in this article, unless otherwise stated.
Reprints and Permissions
About this article
Cite this commodity
Panahi, M.H., Mohseni, M., Bidhendi Yarandi, R. et al. A methodological quality assessment of systematic reviews and meta-analyses of antidepressants event on depression back pain using updated AMSTAR. BMC Med Res Methodol 20, 14 (2020). https://doi.org/10.1186/s12874-020-0903-nine
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s12874-020-0903-ix
Keywords
- AMSTAR 2
- Antidepressants
- Depression back pain
- Meta-analysis
- Systematic review
Source: https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-020-0903-9
0 Response to "Systematic Reviews Explained Amstarã¢â‚¬â€how to Tell the Good From the Bad and the Ugly"
Post a Comment